By Terry Arko
In The Karate Kid, Daniel, a young, bullied kid, befriends a veteran karate master named Mr. Miyagi. Desperate to defend himself, Daniel asks Mr. Miyagi to tell him the “secret” of karate. The answer from Mr. Miyagi is one word, “balance.” Later, Mr. Miyagi explains balance is also the secret to life.
When it comes to pool water chemistry, the secret is also balance. Water is the universal solvent that can dissolve almost anything it comes in contact with. Water is also amphoteric, meaning it naturally seeks to be balanced. Pure water will have a pH of 7.0, which is considered neutral. This is done because the water molecules undergo a process of hydrolysis, which breaks down H2O molecules into H+, an acid, and OH –, a base. These two ions are formed in equilibrium in pure water. Water can function as both an acid and a base because it can donate protons and take protons to balance itself. So, just like Daniel in The Karate Kid, water is always seeking balance.
Water balance values in pools
Five primary values make up water balance in pools. They are as follows:
- Calcium hardness
- Total alkalinity (TA)
- pH
- Temperature
- Total dissolved solids (TDS)
Properly managing and balancing all these values is imperative to ensure optimal pool water disinfection. Also, balanced values are necessary to protect the pool surfaces and equipment from either corrosion or scale formation. All these values are needed to conduct a proper Langelier Saturation Index (LSI). The LSI method matches the five-value test to mathematical factors to determine if the pool water is corrosive, scale-forming, or balanced. In other words, it is a tool to reveal whether the water will corrode or break down metals and cementitious surfaces.
Any negative LSI factors can be considered corrosive. The LSI can also reveal if the water will contribute to damaging calcium carbonate scale to the surface or equipment, especially heat exchangers. Factors that show up on the more positive side are scale forming. The LSI is measured on a scale that goes from -0.5 to +0.7. Balanced water is when the factors total out between -0.3 and +0.3. Ideal balanced water would be when the factors total 0.0. This is the original LSI.
or scaling. Image created with Dall-E by OPENAI
However, recently, within the pool industry, the plus or positive maximum was raised from +0.3 to +0.5. The main reason for this is that slightly scale-forming water is preferred to water that may be corrosive. Corrosion is much more damaging than scale. A small amount of scale on pipes protects against corrosion. According to the American National Standards Institute/Association of Pool and Spa Professionals/International Code Council (ANSI/APSP/ICC)-11 2019, when range chemistry is kept at all maximum ideal values with a temperature of 84 degrees, the LSI is +0.5. The maximum ideal values are pH 7.6, TA-120 parts per million (ppm), CH-400 ppm, and temperature 84 degrees. Cyanuric acid (CYA) and borate can also affect LSI.
CYA impacts the TA test. CYA is a buffer along with TA primarily to keep the pH from decreasing. The LSI is based on the saturation of calcium carbonate. At pool ranges, CYA is not involved in the solubility of calcium carbonate. Only carbonate and bicarbonate are relevant when testing pool water for TA. The alkalinity of the CYA is one-third of the measured CYA level. The following is an example of the CYA/TA correction:
- TA 80 ppm
- CYA 60 ppm/3 = 20 ppm
- 80 – 20 ppm = 60 ppm carbonate alkalinity
- Lowering the TA by 20 ppm also lowers the LSI by 0.1.
Calcium is the lifeblood of water
Of all the values, calcium is the most vital. The calcium must be at least 150 ppm at pool start-up to balance the water. Since LSI is based on the saturation of calcium carbonate, it is evident that calcium must be present to determine proper LSI. Calcium is also needed because it is the one mineral that water craves. Soft water that lacks minerals is termed as aggressive. The aggression is caused by the water looking for calcium to balance itself. Water without calcium will be taken from any source available. In a pool, that could be the plaster or cementitious surface of the pool. A proper amount of calcium in the water is needed to protect the surface and equipment from etching and corrosion. The ideal recommended calcium level for pools is 200 ppm to 400 ppm.
TA the parent to pH

There is often confusion about the relationship between TA and pH. Simply stated, TA is water’s buffering capacity to correct changes in pH.
It is like a parent-to-child relationship. The role of the TA is parental because it guides and manages the pH. As the TA goes, so goes the pH. The TA leads, and the pH follows. A high TA will cause the pH to rise. A low TA will cause the pH to go down. Typically, in pools where the pH tends to decrease, raising the TA by 10 ppm can help keep the pH steady. In scenarios where pH tends to drift up, lowering the TA by 10 ppm can help slow the rise. The recommended ideal levels for TA are 80 ppm to 100 ppm.
Understanding pH
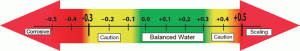
Image courtesy Hasa Pool Inc.
The meaning of pH is the “power of hydrogen.” It represents the concentration of hydrogen ions in water. To fully understand this, one must first look at hydrogen in water. First, remember that pure water is amphoteric because it balances acidic H+ ions and basic OH- ions equally. In pool water, chemicals are either acidic or base, contributing either H+ to lower the pH or OH- to raise the pH. In pools, the ideal pH level is 7.4 to 7.6. To lower pH, acid is used. The most common acid is liquid muriatic acid. Another name for muriatic acid is hydrochloric acid. The process used to create hydrochloric acid is by heating hydrogen chloride. It is important to note that muriatic acid contains hydrogen, and the hydrogen ion lowers the pH. Base products such as soda ash (sodium carbonate) contribute the hydroxyl ion OH- to the water. When the presence of hydroxyl ions supersedes the hydrogen ions, the pH goes up. Acids contribute H+ hydrogen ions that cause the pH to decrease, and bases contribute OH- hydroxyl ions that cause the pH to rise.
The current problem of high pH

The most prevalent water balance challenge lately has been the continual pH increase in pools. There are plenty of theories to explain this. The author believes that the primary cause of increased pH is the new accoutrements being added to pools. These include fountains, waterfalls, negative edges, raised spas, and bubblers. These items increase turbulence at the water’s surface, increasing aeration and leading to carbon dioxide (CO2) leaving the water.
When CO2 leaves the water, carbonic acid (H2CO3) is replaced by a bicarbonate ion (HCO3 -), and the bicarbonate ion is replaced by a carbonate ion (CO3 2-). During this process, hydrogen H+ dissociates until it is consumed. There is less H+, and so the pH increases.
Another device that increases pH is a saltwater generator (SWG). An SWG uses salt water that passes through a unit with positive and negative metallic cells. At the positive cell, chlorine gas is created, and at the negative cell, hydrogen gas forms. These gases cause turbulence and aeration, which causes CO2 to leave the water, which consumes hydrogen and causes the pH to go up.
Hypochlorite forms of chlorine do not raise pH

It has been theoretically presented that these sanitizers lead to a high pH because of the high-pH ingredients in liquid sodium hypochlorite and calcium hypochlorite. The incorrect aspect of this is instructing the use of acid whenever these forms of chlorine are added. This is an erroneous scenario based on incomplete assumptions. The pH of liquid sodium hypochlorite is 13, and calcium hypochlorite is 11. When liquid sodium hypochlorite is added, the immediate addition of sodium hydroxide causes the pH to increase.
Calcium hypochlorite contributes to calcium hydroxide, leading to an immediate pH rise. However, this is only half of the story. Once again, thanks to the hydrolysis of water and the effects of UV sunlight and oxidation, the HOCl created in the water is broken down to HCl or hydrochloric acid. The amount of HCl formed is in equilibrium with the amount of sodium hydroxide or calcium hydroxide, which leads to a net zero change in the pH. Suppose high pH is an issue when using hypochlorite sanitizers. In that case, it is more likely a result of high TA or too much aeration from devices such as fountains or waterfalls.
The role of temperature in water balance
Now, the discussion turns to temperature, which is identified as a crucial component of the LSI and water balance. Initially, it is understood that LSI is predicated on the saturation of calcium carbonate in water. The role temperature plays is very distinct. Calcium carbonate is soluble in colder water; therefore, it is not visible nor in a state where it can form scale.
Calcium becomes less soluble as the water heats up and precipitates as a solid to create scale. This metamorphosis from soluble to insoluble leads to a change in the LSI. This also causes an increase in pH, and the increased pH with the insoluble calcium leads to increased calcium hardness, cloudy water, and scale. A 15-degree increase in temperature increases the LSI by 0.1.
TDS the big picture

TDS is one of the final determiners of balanced water and sufficient pool water disinfection. Every chemical or compound, including bather waste and environmental debris, gets broken down into dissolved solids. These materials are unseen, and the smallest microns cannot be removed by filtration. Therefore, they continually build up, taking water from an unsaturated state to a saturated state. When TDS reaches an extreme of 1500 ppm over the start-up water, it will consume much more free chlorine. A higher TDS can cause the consumption of up to 50 per cent more free chlorine than in a newly filled pool.
High TDS also leads to higher pH, alkalinity, calcium hardness, poor disinfection, cloudy water, swimmer irritation, and extreme scale formation. TDS is one of the most critical tests for quality water balance. A TDS test of both the pool and the source water should be done regularly to determine when the pool water needs to be drained and diluted. The best practice is to know the source water TDS upon the pool’s start-up fill.
Whenever the pool water reaches 1500 ppm over the fill, it should be partially drained and diluted to return the TDS as close as possible to the fill water. This practice will lower TDS and CYA levels and organic-bound chloramines, calcium, salts, and phosphates. Dilution is the solution in a big way when it comes to TDS.
Balance is the secret of water chemistry. Understanding the relationship between all the values and properly controlling the LSI is the best practice for clean, clear water and happy swimmers.
 Terry Arko is a product training and content manager for HASA Pool Inc., a manufacturer and distributor of pool and spa water treatment products in Saugus, Calif. He has more than 40 years of experience in the pool and spa/hot tub industry, working in service, repair, retail sales, chemical manufacturing, technical service, commercial sales, and product development. He has written more than 100 published articles on water chemistry and has been an instructor of water chemistry courses for more than 25 years. Arko serves as a voting member on the Recreational Water & Air Quality Committee (RWAQC) board. He is a Commercial Pool Operator (CPO) course instructor, a Pool Chemistry Certified Residential course teacher for the Pool Chemistry Training Institute (PCTI), and a Pool & Spa Marketing Editorial Advisory Committee member. Arko can be reached at [email protected].
Terry Arko is a product training and content manager for HASA Pool Inc., a manufacturer and distributor of pool and spa water treatment products in Saugus, Calif. He has more than 40 years of experience in the pool and spa/hot tub industry, working in service, repair, retail sales, chemical manufacturing, technical service, commercial sales, and product development. He has written more than 100 published articles on water chemistry and has been an instructor of water chemistry courses for more than 25 years. Arko serves as a voting member on the Recreational Water & Air Quality Committee (RWAQC) board. He is a Commercial Pool Operator (CPO) course instructor, a Pool Chemistry Certified Residential course teacher for the Pool Chemistry Training Institute (PCTI), and a Pool & Spa Marketing Editorial Advisory Committee member. Arko can be reached at [email protected].
The post Balanced chemistry: The secret behind pool longevity appeared first on Pool & Spa Marketing.








